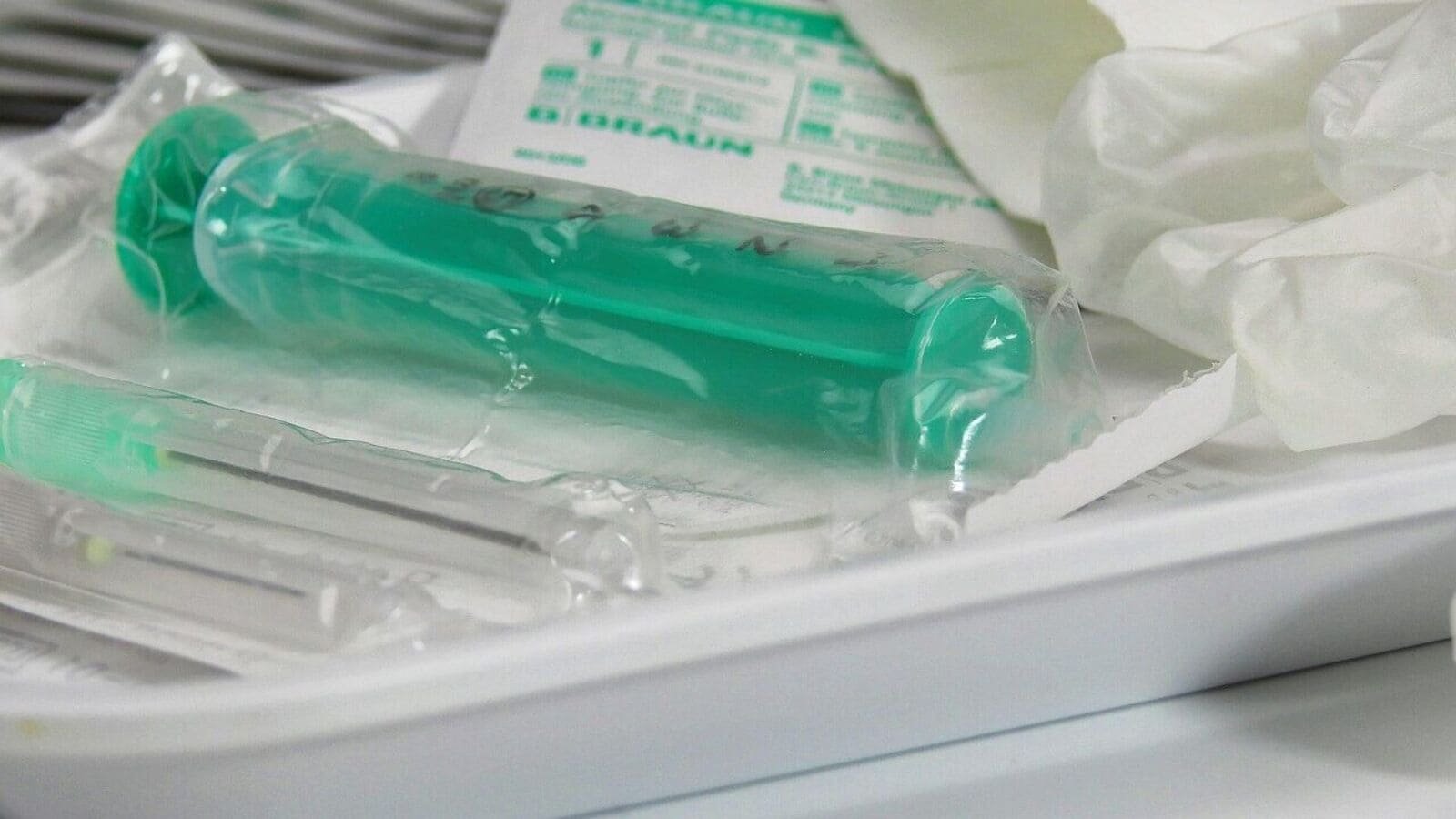
New Delhi: The government liberated pharmaceutical polyethylene with high density (HDPE), critical raw material used in the production of disposable syringes and IV cannals, compulsory quality certification according to Indian standards (BIS).
The aim of this step, which was announced by the Ministry of Chemicals and Fertilizers, is to prevent disruption of supplies in the medical sector in the middle of the fear that the compulsory BIS standards have been reached by the import of HDPE EP/USP. These specialized stamps, mostly from overseas, are not produced on the domestic market in a scale and are necessary to ensure continuous production of basic medical equipment.
“Given the sensitivity of the raw materials needed for the production of syringes, the government has taken this step on the basis of its market intelligence. The industry also asked,” said a higher government official.
The exception applies specifically to the HDPE EP/USP used in Plungy Syders and IV components. It does not apply to use in packaging or other plastic products that will still require BIS certification.
The change in the order to control the quality of 2022 issued the Ministry of Chemicals and Petrochemicals and proceeded in consultation with BIS. According to the announcement, it forgets the earlier clause and adds a new exception in the public interest.
The decision comes in the middle of a sharp decrease in HDPE imports. According to the Ministry of Trade, HDPE imports dropped by almost 42% to $ 1,151.23 million in FY25 of $ 1,979.39 million in FY24. Compared to FY23, imports decrease by 34%, reflecting the impact of regulatory narrow places.
Industry management welcomed this step and said it offered early relief to manufacturers who depend on the imported HDPE with EP/USP certification, especially for exports to firmly regulated markets.
“This is a practical decision of the government. Any delay or disruption of access to polyethylene Class EP/USP may affect the syringe and IV established production, especially with increasing demand for health care,” said an executive in the exporter of medical devices.
India mainly imports HDPE medical classes used in syringes and IV sets from South Korea, Singapore, USA, Saudi Arabia and Germany.
(Tagstotranslate) HDPE liberation India





